Diethyl Ether And Peroxides

Storing diethylether in a brown bottle over sodium hydroxide.
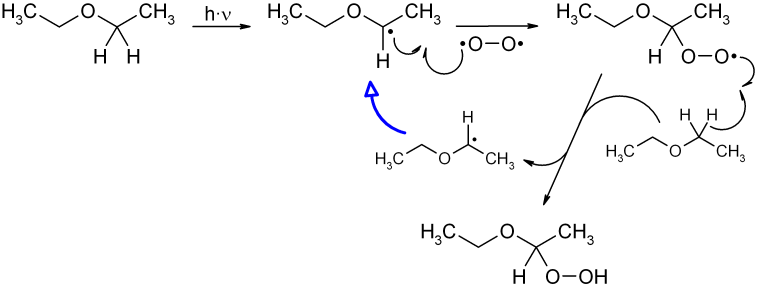
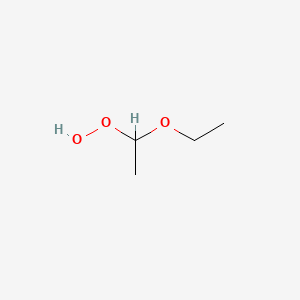
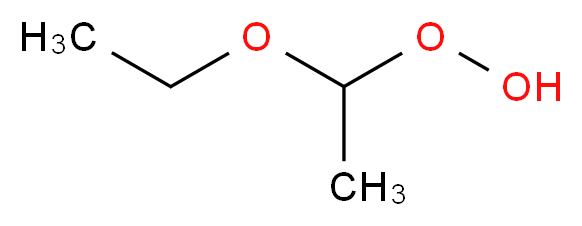
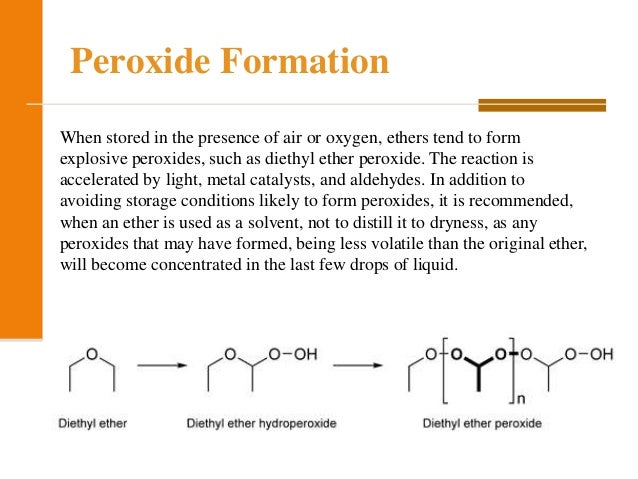
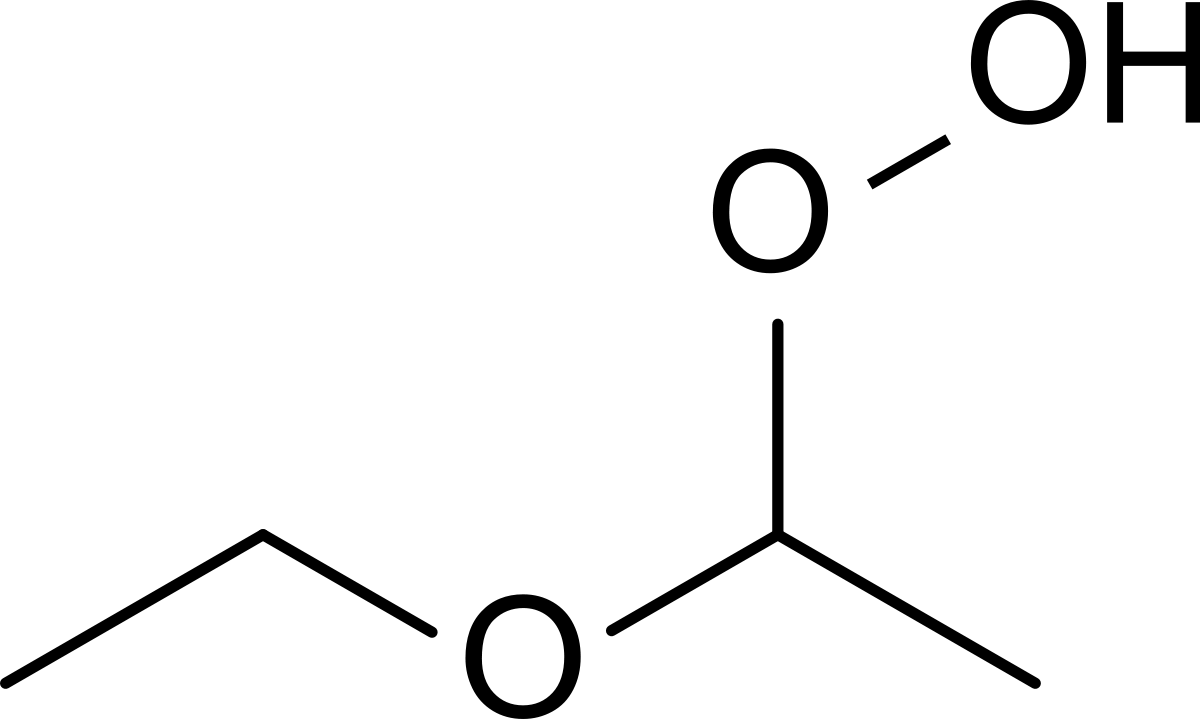
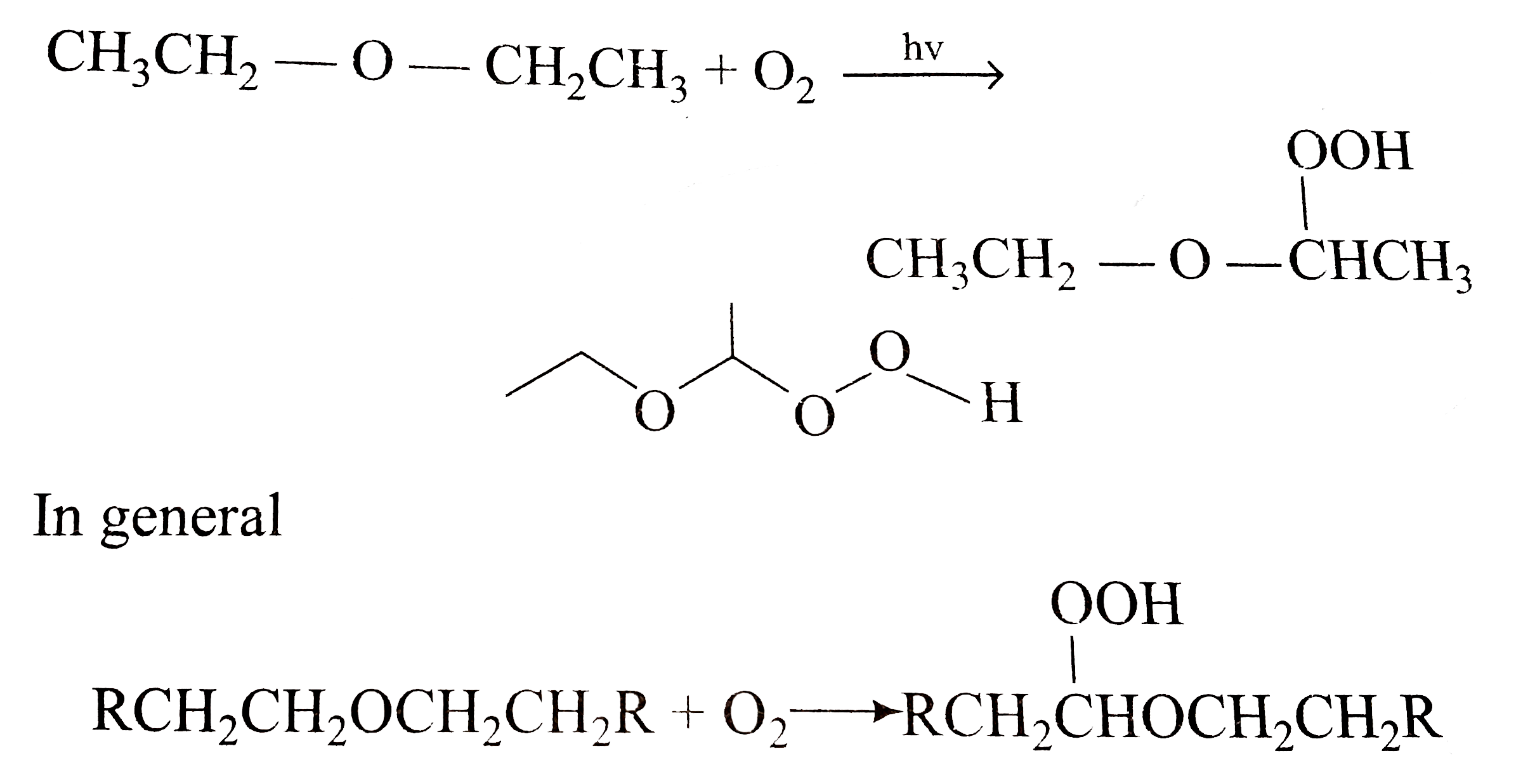
Diethyl ether and peroxides. If you prevent the formation of diethylether hydroperoxides by. Water and peroxides can be removed by either. Diethyl ether peroxide also known as ethylidene peroxide is a polymerization product of diethyl ether hydroperoxide. Ether peroxides are higher boiling and are contact explosives when dry.
It was formerly used as a general anesthetic until. Diethyl ether hydroperoxide and its condensation products are blamed for the explosive organic peroxides that slowly form upon exposure of diethyl ether to ambient air and temperature conditions. Storage over naoh precipitates the intermediate ether hydroperoxides. Diethyl ether is typically supplied with trace amounts of the antioxidant bht 2 6 di tert butyl 4 methylphenol which reduces the formation of peroxides.