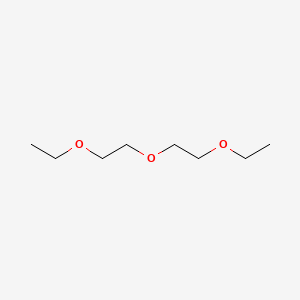
Diethyl Ether And Oxygen Reaction
Oxidation an ether will slowly react with oxygen in the air to form explosive peroxides.
Diethyl ether and oxygen reaction. The main route of exposure is inhalation. Topic ether chemical properties of ether in this video explain the organic chemistry take the ether and air or oxygen to form products and also check the compound present the ether peroxide. Cleavage then involves the nucleophilic attack by a halide ion on this protonated ether with the displacement of the weakly basic alcohol molecule. Chemical properties of diethyl ether c 2 h 5 2 o.
Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then replaced by other substances in the 1960s. It was formerly used as a general anesthetic until. The oxygen of an ether is basic similar to the oxygen of an alcohol. It is known that the oxygen atom of ether is basic similar to the oxygen atom of alcohol.
Diethyl ether was the major oxygenate produced at all ethyl oxygen ratios and the peak temperature for ether evolution varied from 220 to 266. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight. The initial reaction between an ether and an acid is no doubt the formation of the protonated ether. C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o.
Diethyl ether peroxide is the peroxide that is formed when oxygen reacts with diethyl ether. Diethyl ether cas 60 29 7 is a component of starting fluids and is used as a solvent in the manufacture of synthetic dyes and plastics. This alcohol further reacts with halide to form a second mole of alkyl halide and water. Finally the oxygen which is now part of a protonated ether loses the proton to give the final product diethyl ether.
For example the reaction of dialkyl ether produces initially an alkyl halide and alcohol. In addition to butane a number of oxidation products including diethyl ether ethanol acetaldehyde surface acetate ethylene carbon dioxide and water were formed on the oxygen dosed ag 110 surface.