Boiling Point Of Diethyl Ether Ch3ch2och2ch3

Citation needed ether is sensitive to light and air tending to form explosive peroxides.
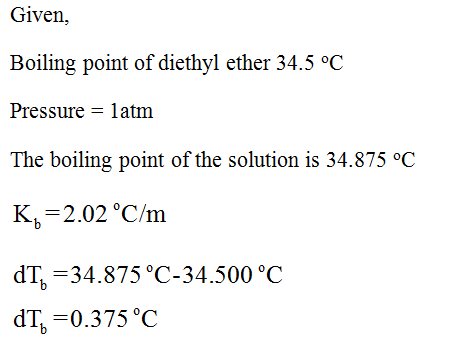
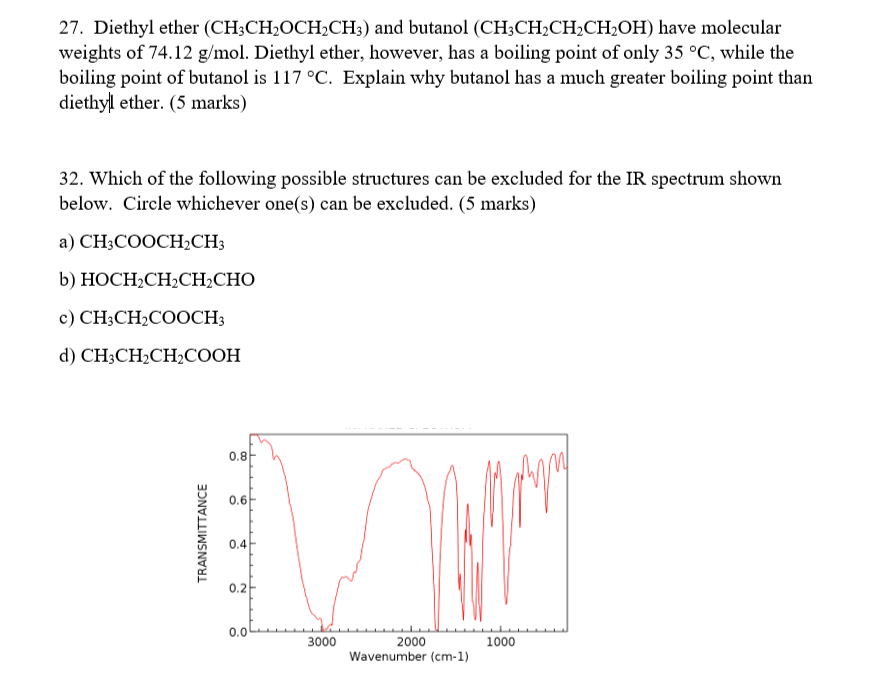
Boiling point of diethyl ether ch3ch2och2ch3. The boiling point of diethyl ether ch3ch2och2ch3 is 34 50 c at 1 atmosphere and its kbp value is 2 02 c m. Given the molecules diethyl ether ch3ch2och2ch3 and 1 butanol ch3ch2ch2ch2oh 1 butanol ch3ch2ch2ch2oh has the higher boiling point mainly due to hydrogen bonding influences. Examples include benzene ce c6h6 carbon tetrachloride ce ccl4 and diethyl ether ce ch3ch2och2ch3. 1 butanol ch3ch2ch2ch2oh and diethyl ether ch3ch2och2ch3 both have the same molecular formula but the boiling point of 1 butanol is.
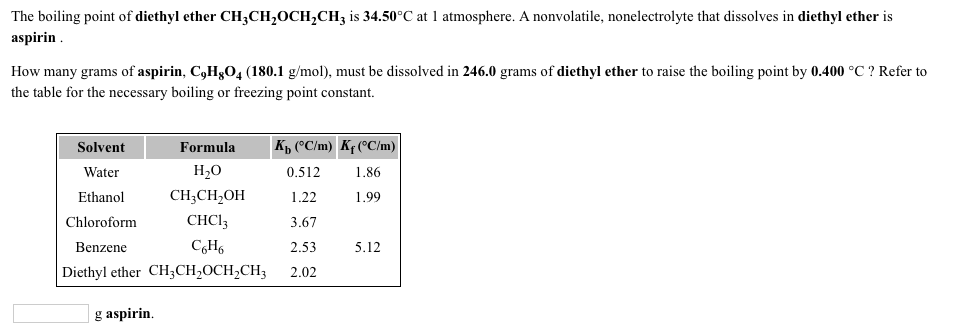
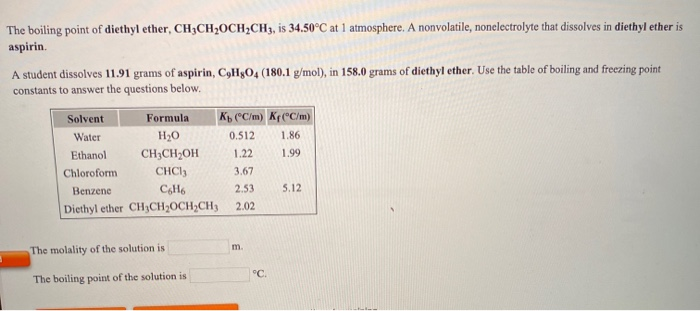
Ether peroxides have a higher boiling point than ether and are contact explosives when dry. Question asked dec 26 2019. A nonvolatile nonelectrolyte that dissolves in diethyl ether is aspirin. 1 the boiling point of diethyl ether ch3ch2och2ch3 is 34 500 c at 1 atmosphere.
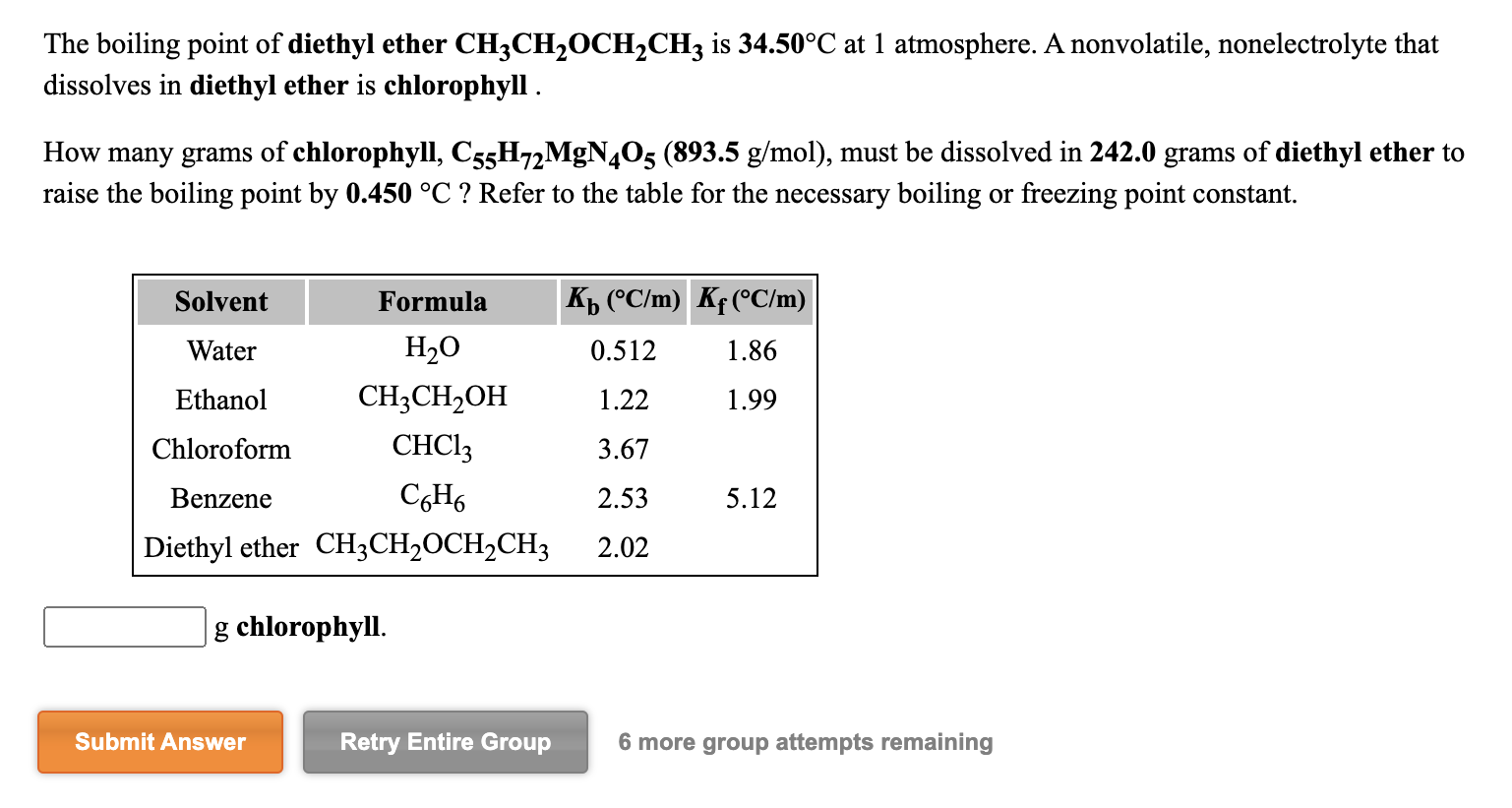
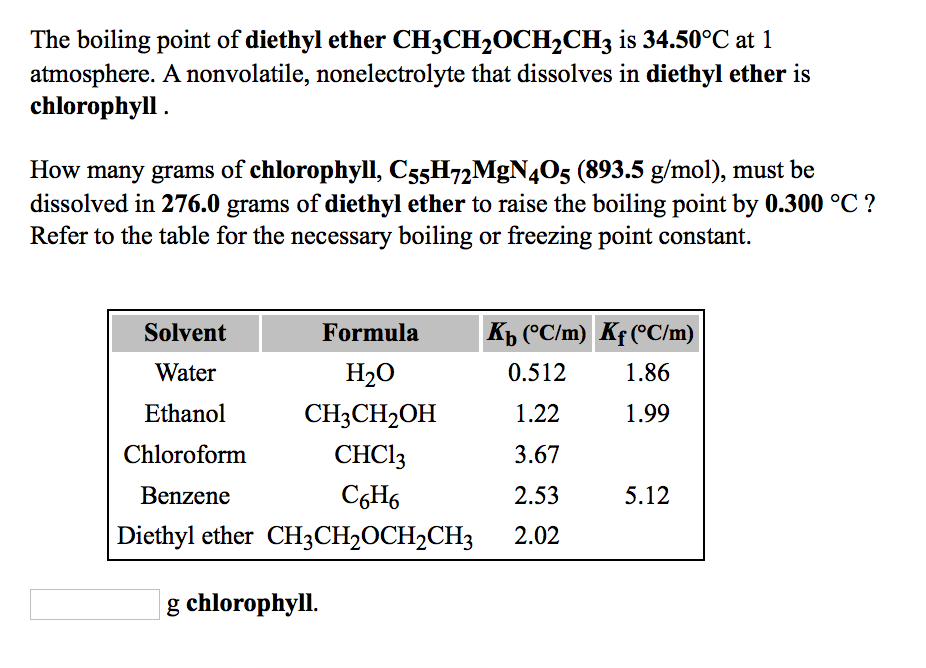
Kb diethyl ether 2 02 c m in a laboratory experiment students synthesized a new compound and found that when 11 99 grams of the compound were dissolved in 275 4 grams of diethyl ether the solution began to boil at 34 598 c. 1 the boiling point of diethyl ether ch3ch2och2ch3 is 34 50 c at 1 atmosphere. The boiling point dipole moment and dielectric constant of each solvent is included. A nonvolatile nonelectrolyte that dissolves in diethyl ether is chlorophyll.
All of these solvents are clear colorless liquids. Explain why diethyl ether ch3ch2och2ch3 and butan 1 ol ch3ch2ch2ch2oh have similar solubility properties in water but butan 1 ol has a much higher boiling point. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. How many grams of chlorophyll c55h72mgn4o5 893 5 g mol must be dissolved in 299 0 grams of diethyl ether to raise the boiling point by 0 500 c.
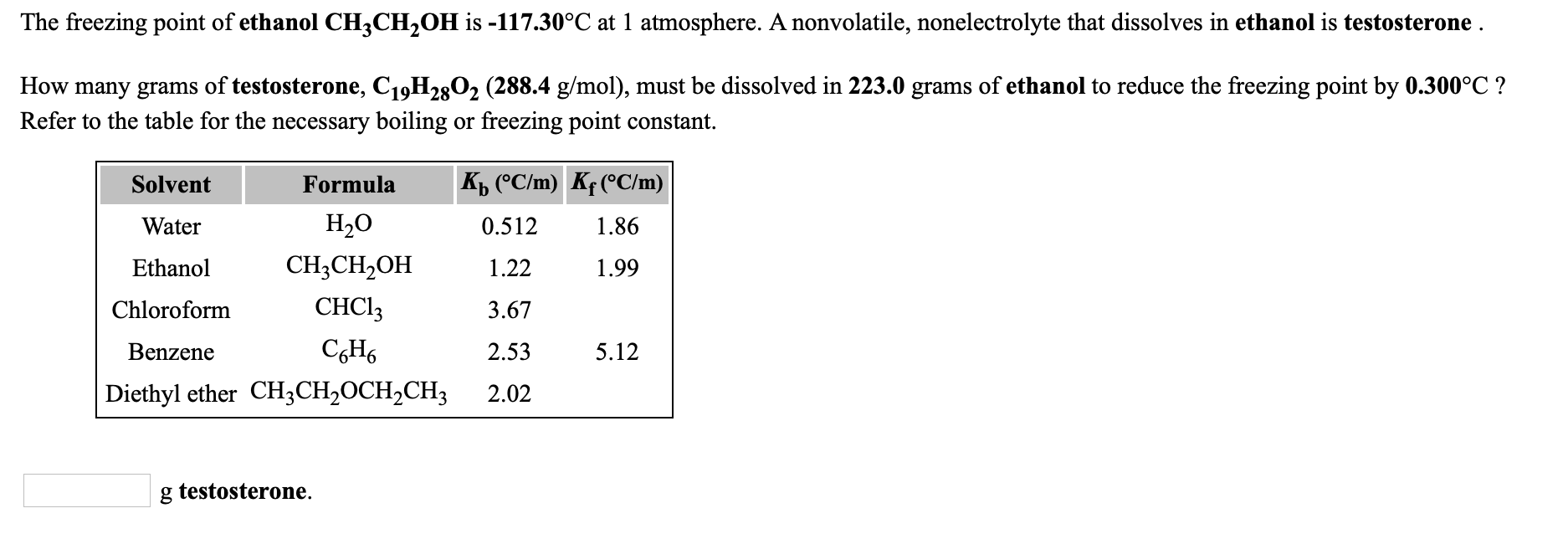
The boiling point of diethyl ether ch3ch2och2ch3 is 34 500 c at 1 atmosphere. How many grams of aspirin c9h8o4 180 1 g mol must be dissolved in 200 0 grams of diethyl ether to raise the boiling point by 0 300 c. Kb diethyl ether 2 02 c min a laboratory experiment students synthesized a new compound and found that when 14 04 grams of the compound were dissolved in 299 9 grams of diethyl ether the solution began to boil at 35 025 c. The diffusion of diethyl ether in air is 9 18 10 6 m 2 s 298 k 101 325 kpa.