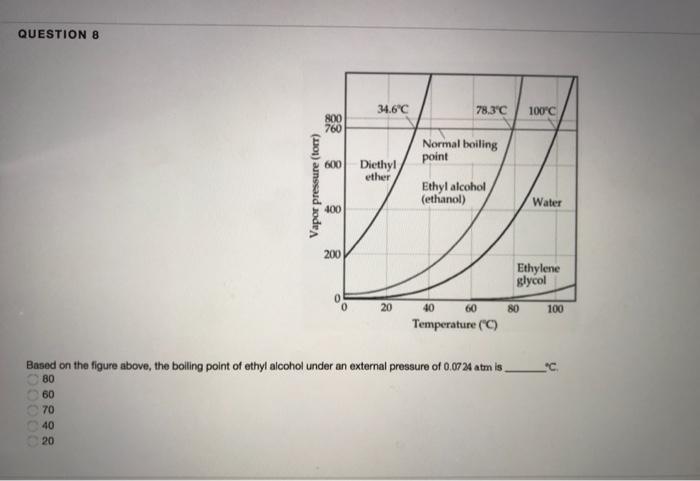
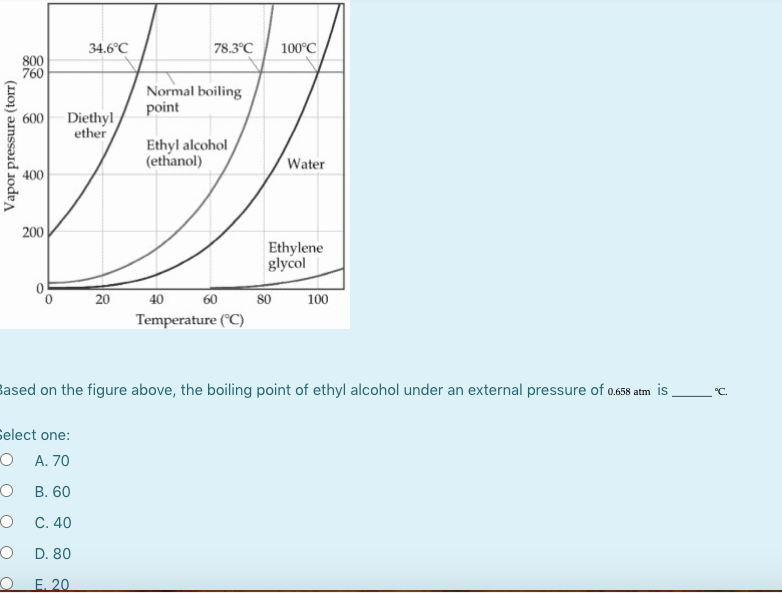
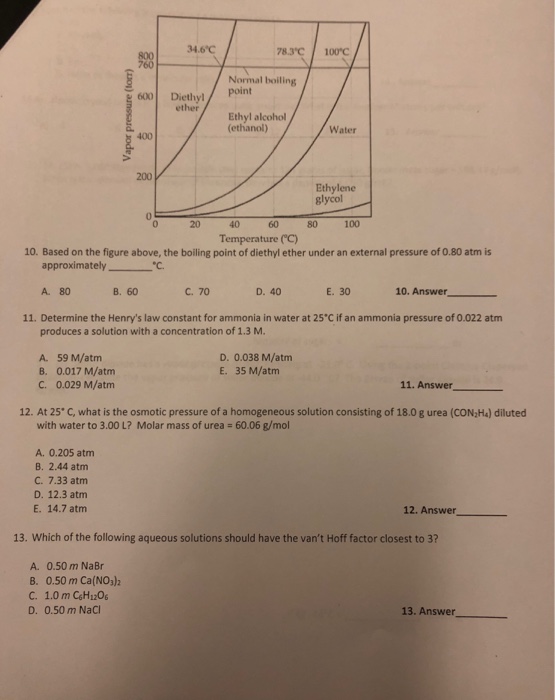
Boiling Point Of Diethyl Ether At 1 Atm
Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight.
Boiling point of diethyl ether at 1 atm. It is highly recommended that you seek the material safety datasheet for this chemical from a reliable source such as siri and follow its directions msds for diethyl ether is available at mallinckrodt baker. Chemical properties of diethyl ether c 2 h 5 2 o. Combustion ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water. Citation needed ether is sensitive to light and air tending to form explosive peroxides.
The handling of this chemical may incur notable safety precautions. Ether peroxides have a higher boiling point than ether and are contact explosives when dry. Boiling point 34 6 c hvap 34 6 c 26 5 kj mol specific heat. C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o.
The following information is given for ether c2h5oc2h5 at 1atm. Diethyl ether c2h5 o c2h5 ether ethyl ether anesthesia ether ethyl oxide bp 35 c. Methanol diethyl ether 65334 65 8 chemsrc provides diethyl ether cas 60 29 7 msds density melting point boiling point structure formula molecular weight etc. Boiling point 34 6 ã â c hvap 34 6 ã â c 26 5 kj mol specific heat liquid 2 32 j gã â c at a pressure of 1 atm kj of heat are needed to.
The diffusion of diethyl ether in air is 9 18 10 6 m 2 s 298 k 101 325 kpa. The following information is given for ether c2h5oc2h5 at 1atm.